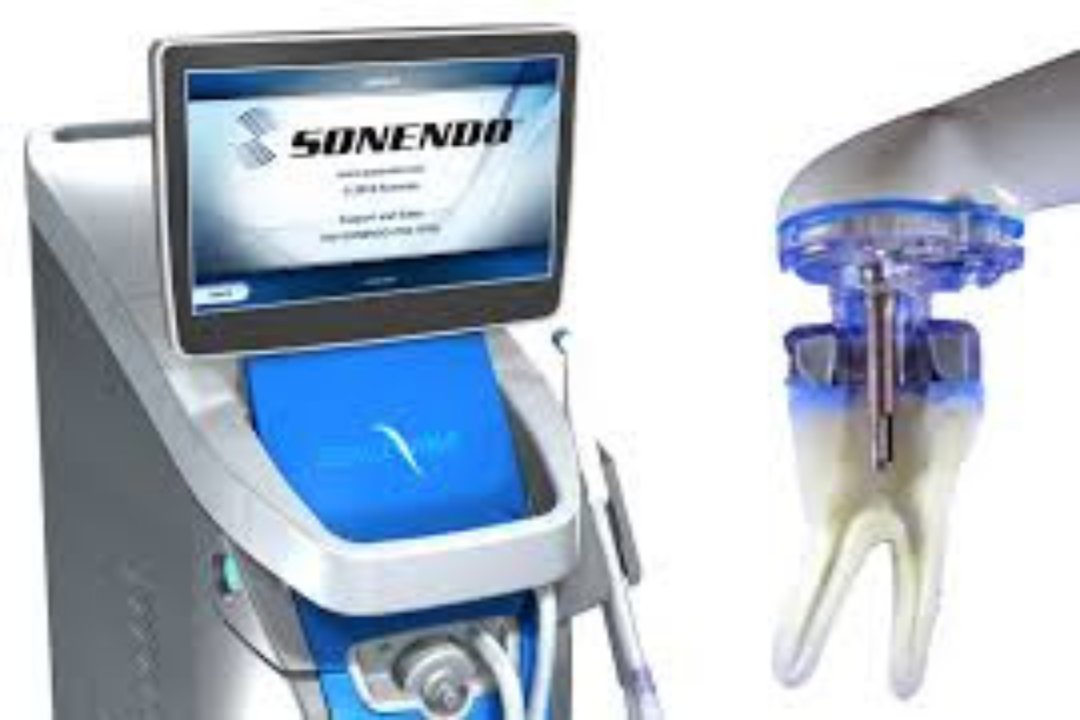
We have had some recent questions about a new technology for cleaning and disinfecting the root canal system that is being heavily marketed in the dental community. This system, known as the GentleWave System, by Sonendo Inc. (Laguna Hills, CA), consists of a console that is used to deliver the traditional irrigating solutions through a proprietary treatment instrument (TI). The tooth is accessed, canals are located, patency established and canals shaped to #20 .07 taper, the TI is sealed to the access of the tooth. The console then systematically delivers the irrigating solutions through the tip of the TI. Through the TI, irrigating solutions are delivered in a “degassed” state (no air bubbles) using acoustic and hydrodynamic cavitation. The solutions are simultaneously removed using built-in vented suction through the same TI.
- the technique provides improved removal of organic matter (pulp tissue and biofilm) a “higher standard of clean”
- the technique allows for minimal instrumentation of the canals, preserving valuable tooth structure
- the technique allows for improved cleaning of canals, lateral canals, isthmus
- 97% success rate of endodontic cases – in initial clincial studies
Like many new technologies and materials that are introduced in dentistry, much of the research supporting the new technology or product comes from the manufacturer or studies that are supported by the manufacturer. It is well known that studies supported by a manufacturer tend to have bias that favors the funding source known as funding bias. As clinicians, it is up to us to evaluate the research that is presented to us as we make important decisions.
- Design Bias: Bias that occurs when the design of a study selects or encourages a specific outcome. This is 12 month prospective study with no controls. How easy would it have been to make this a randomized, double blinded study and compare this new technology to the conventional endodontic approach. Then we might have a better idea if this technology actually improves the outcomes of treatment. Design bias is also evident in the discussion of this study by its failure to acknowledge the one-sided study design and in the analysis of what the results mean (see below)
- Attrition Bias: Systematic error introduced into a study by loss of participants: 75 of the 89 patients in the study returned for the 12 month follow-up. This is a drop-out rate of 15.7%. A major problem with almost all of our medical/dental studies is that we make assumptions about that 15%. Since we don’t have information on them, we exclude them from the study results. So when the study says they have 97.3% success rate – they are assuming that the 15.7% that dropped out were also successes.
- Selection/Sampling Bias: Bias introduced by the selections of participants: 72 of the 89 patients included in this study were diagnosed with irreversible pulpitis. Only 14 of 89 patients in the study had a necrotic pulp. Cases of irreversible pulpitis have the highest success rates in endodontics. To heavily weight a study with irreversible pulpitis cases creates a significant bias. A study focused on treating necrotic pulpal tissue would have been much more meaningful – especially if the study aims to prove that the GentleWave procedure will better remove the microbiota from the canal system.
- Sponsorship Bias: Refers to the overwhelming tendency of a scientific study to support the interests of the study’s financial sponsor. While the financial sponsorship is noted in the footnotes of the article, the tendency toward this bias requires a stricter methodology and implementation to reduce bias and a more clear and open discussion regarding analysis of results and clinical interpretation of these results.
- Citation Bias: The success rate of this study (comprised mostly of irreversible pulpitis cases) is then compared to the success rates of other previous studies (comprised of teeth with periapical radiolucencies – which means necrotic cases) as an argument of improved cleaning and efficacy is an example of selection and citation bias together.
- Internal Validity Bias: Refers to the author and reviewer’s confidence that the study design, implementation and data analysis have minimized or eliminated bias. There appears to be no effort on the part of the authors to evaluate or report any bias in the study design, implementation or data analysis.
- External Validity Bias: Refers to the degree to which the study’s design allow its findings to be able to be generalized to other groups or populations. The failure in methodology of this prospective study without positive or negative controls, make the comparisons made in the discussion in regards to improved clinicial efficacy unsubstantiated.
- The discussion regarding single visit vs. multiple visit endodontic treatment is again not a fair comparison when 89% of the cases in the study were irreversible pulpitis. Those who advocate for multiple visit endodontic therapy are recommending that for necrotic cases and usually treat irreversible pulpitis in single visit as well.
- The discussion about reduced post-operative pain is again biased by a study heavily weighted with irreversible pulpitis.
In order to substantiate the claims being made by the manufacturer, we need randomized, double-blinded, controlled clinical trials focusing on necrotic teeth. With this kind of data, we would be able to better evaluate the effectiveness of the GentleWave procedure over conventional endodontic therapy.
In our practice at SSE, we are committed to providing the highest levels of endodontic care. If any technology can demonstrate a significant improvement over conventional approach with serious randomized, controlled studies, rather than cohort studies, case reports and in vitro studies, we will be interested in incorporating that technology. We look forward to learning more about the GentleWave procedure in an upcoming office demonstration. We will also be watching for better scientific evidence to be published and will do our best to keep you up to date with our findings.